- March 14, 2025

If you were to sift through the dirt in your backyard, you probably wouldn’t find much more than a few worms or maybe an interesting rock. But what you may not know is that there are millions of microbes in your fistful of dirt — fungal spores or bacteria you can’t even see — that might help researchers develop new drugs to fight disease.
Dr. Elizabeth Kaweesa has learned to value these microbes and their hidden natural products the way some people might value their cats. Kaweesa grew up in Uganda surrounded by tropical herbs and plants, but it wasn’t until she was taking a chemistry class in college when she realized how humans have been using natural products to treat all kinds of aches and sicknesses for centuries. Now Kaweesa is finishing her PhD at the University of Florida’s Whitney Laboratory, in Flagler County, where she studies microbes to tease out their medicinal secrets and to identify products for developing new drugs. Kaweesa’s graduate dissertation investigates mensacarcin, a chemical produced by bacteria (discovered in the dirt outside a café in Germany) that happens to kill cancer.
The road to developing a new treatment for cancer can be full of twists and turns. First, a compound needs to be discovered, then extracted and purified. In Kaweesa’s case, this looked like receiving soil samples and growing bacteria in the lab. She broke the bacteria apart to isolate mensacarcin, and this isolated compound was then administered to an array of cancer cells representing the most common types of human cancer.
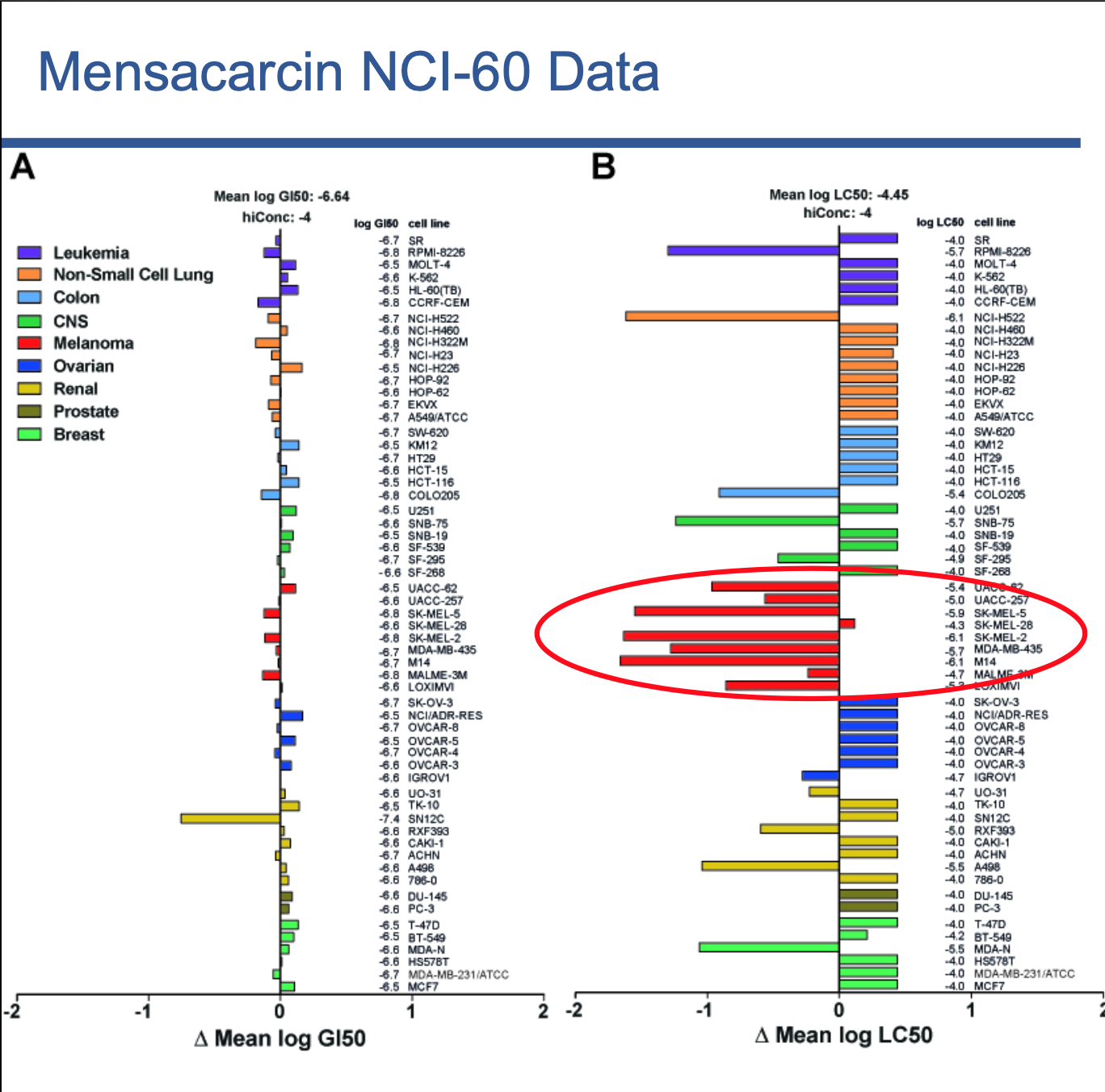
After treating different cell lines with mensacarcin at the National Cancer Institute, Kaweesa could determine if it targets a specific type of cancer. She shows her data with a bar graph where each collection of colored bars represents a specific cancer — purple for leukemia, turquoise for colon cancers, lime-green for breast cancers, etc. In her figure, a collection of red bars is circled, distinguished from the rest. The news in those red bars is exciting — mensacarcin targets melanoma, or skin cancer.
“When you’re developing a potential drug, you want something specific,” Kaweesa explained. “If we have a drug that kills every type of cancer, chances are we’d be wiping out all the good cells, too. Mensacarcin appears to be toxic only to melanoma cells, which means we should be able to use it to develop a drug that targets melanoma specifically, reducing the risk of unintended side effects.”

Once mensacarcin was shown to target skin cancer cells, Kaweesa tested the compound to identify disruptions in molecular activity that pinpoint how mensacarcin kills skin cancer cells. Kaweesa’s results show that mensacarcin impairs mitochondrial function and disintegrates the nuclei in melanoma cells, effectively suffocating them and scrambling their brains. And, while mensacarcin doesn’t kill other types of cancer, it does appear to slow their growth, meaning that mensarcarcin could be used in combination with other drugs to improve treatments of other types of cancer.
Currently, the most common treatment for skin cancer is a drug called Vemurafenib. In a little over three months after starting Vemurafenib treatments, a patient’s body can go from being pockmarked with tumors to having smooth, healthy skin. Unfortunately, the cancer often becomes resistant to the treatment, and the tumors return.
That’s why discovering new treatments is so important in the fight against skin cancer. If patients who have developed resistance can respond to new drugs, then their chances of recovery increase.
“We’ve shown in the lab that mensacarcin can kill Vemurafenib-resistant cancer cells,” Kaweesa said. “I was shocked when I saw these results because these cells are extremely resistant to most treatments, so I really didn’t expect them to respond to mensacarcin.”
To date, the effectiveness of mensacarcin has only been tested in cell cultures and has not yet been demonstrated in live animals. This step is crucial before mensacarcin can be considered for use in human patients.
Production poses another hurdle. “One liter of bacteria culture only produces between 40–60 milligrams of mensacarcin,” Kaweesa said. “That’s not a lot, so the next step will be to figure out a way to produce more, whether through the bacteria itself or through synthetic production. But that’s beyond my expertise,” she explained with a smile. “Future work will require collaboration with other labs.”
Kaweesa’s research highlights how nature produces some of the most efficient cancer-killing drugs. And these drugs are hiding in some of the most exotic—or sometimes mundane — locations on the planet. From the slimy mucus coating fish, to marine sponges, to bacteria in the dirt in our own backyard — we just need to figure out where to look.