- November 23, 2024
-
-
Loading

Loading
I heard a story once of a toddler who leaned over too far in her chair in the kitchen and toppled off. The chair landed on her little toe, slicing it cleanly from her foot. When her parents rushed her to the hospital, the doctor reassured them that, at this child’s age, her little toe could regrow back normally. You may have heard similar stories throughout your lifetime and wondered: Why can’t our bodies do that when we’re older?
Now, with the help of a little-known marine worm found in the muddy estuaries right here in Flagler and St. Johns counties, scientists at University of Florida’s Whitney Laboratory for Marine Bioscience are beginning to piece together the answer to that question and learn how regenerative abilities can change over time.
“An animal’s capacity for regeneration can vary throughout its life,” explained Alicia Boyd, a graduate student at Whitney Laboratory studying regeneration. “One classic example comes from a frog, which has remarkable regenerative potential as a tadpole, but, with age, that potential is lost.”
What’s exciting is that the reverse process can also be true.
The research represents important early steps for understanding the basic biological principles of regeneration and restoring regenerative potential when and where it has been lost. The hope is to someday apply these principles to other species — maybe even humans — to help us regenerate tissues that don’t regenerate on their own.
“There are other regenerative models that seemingly gain regenerative potential as they develop,” Boyd said, “one of them being the animal we study in our lab, Capitella teleta.”
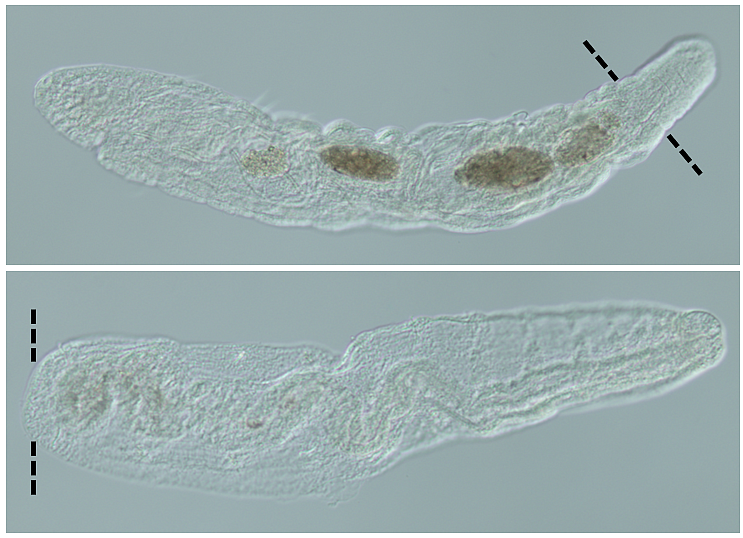
Capitella is an annelid, a segmented worm not unlike the kind you might stick on the end of your fishhook. These worms live out their lives in muddy estuaries and transition from tiny torpedo-shaped swimming larvae to a burrowing worm in a process called metamorphosis. To date, researchers assumed that only adults were capable of regeneration, albeit in a limited way: as adults, Capitella can regenerate posterior fragments (i.e. its tail) but not anterior fragments (i.e. its head).
“The DNA content is the same throughout the worm,” explained Lauren Kunselman, another graduate student studying regeneration at Whitney. “This means that differences in regenerative ability in Capitella is not based on the presence or absence of genes, but something else, such as changes in gene expression.”
This difference in regenerative ability in Capitella allows scientists to research the most fundamental questions of regenerative biology that may be applied to human health: When does regenerative potential begin and how might it change during a life cycle? Why might an animal regenerate one way but not another?
Boyd became interested in the topic of regenerative potential after having a simple conversation in the lab with her supervisor, Dr. Elaine Seaver. As Boyd was sorting worm larvae, she asked Dr. Seaver whether the larvae were capable of regenerating like the adults. Dr. Seaver’s response was essentially a shrug—besides a few inconclusive preliminary experiments, no one had seriously tried answering that question before now.
The experiments that address these questions yield exciting results. Boyd cut the worms using specific landmarks—pigments that mark the worm’s eyes and wavy thread-like appendages called cilia that wrap around specific regions of the animal’s trunk.
“These features let us make amputations at consistent locations in the body,” Boyd explained. This consistency is crucial to weed out variation between animals that may make the results difficult to interpret.
After making the cuts, Boyd assessed the regenerative ability of her larvae.
“There are three main steps to regeneration,” Boyd said. “Wound healing, cell proliferation, and the replacement of lost tissues.” Wound healing is essentially the closing of a wound to prevent infections and leaking body fluids; this process results in the formation of a continuous layer of cells. Cell proliferation produces new cells where the wound occurs — in regenerating animals, tissue may be “remade” from cells in the region of the wound site, or they may be created from a separate pool of stem cells. The replacement of lost tissues is assessed by the regrowth of structures that were removed during amputations.
“It looks like Capitella larvae can start regeneration by wound healing and generating new cells,” Boyd said, “but they can’t reliably replace lost tissue.” In essence, the worm larvae can start regeneration, but they can’t finish it.
However, there’s a plot twist to this story that brings an excited smile to Boyd’s face whenever she talks about it.
“One of the features we looked at was the regeneration of the brain and eyes in our larvae,” Boyd said. “They weren’t able to regrow their brains, but it does look like some animals were capable of regenerating their eyes.” Boyd is quick to point out that the numbers were very small—of 640 cases, only 36 instances of eye regeneration occurred.
“This is a very surprising finding. Perhaps if we can tweak the conditions, we can increase the proportion of times that eyes reform or even facilitate the regeneration of other structures such as the brain."
ELAINE SEAVER
“This is a very surprising finding since it is the first evidence of anterior regeneration in Capitella at any stage of its lifespan,” said Dr. Elaine Seaver, the principal investigator supervising this research and one of the world’s leading experts on Capitella. “No one else has looked at this so thoroughly, and it is a testament to Alicia’s perseverance to discover such a rare occurrence. Perhaps if we can tweak the conditions, we can increase the proportion of times that eyes reform or even facilitate the regeneration of other structures such as the brain.”
“The main takeaway here is that context is critical,” Boyd explained. “Regeneration is dynamic and can be gained or lost within an organism’s lifecycle. Understanding when and where regeneration is possible is crucial for expanding regenerative abilities where it might be absent.”
Boyd intends to follow up with this research by looking to see if stem cells might explain the difference between regenerative potential in Capitella larvae and adults.
“The next question one might begin to wonder is what is responsible for differing regenerative ability,” Kunselmen said. “That’s essentially what my research is doing, figuring out the ‘why’ behind regenerative potential by looking at tissue that is good at regeneration compared to tissue that is unable to regenerate.”
So far it looks like a group of genes known as Wnt (rhymes with hint) play an important role.
During development, Wnt genes help ensure the head and tail go where they’re supposed to go and form when they’re supposed to form, sort of like shift supervisors assigning specific rooms, times and tasks to their employees.
“The Wnt gene family has also been shown to play an important role in regeneration,” Kunselman said. “Usually, when it is downregulated, it promotes anterior growth, and when it is upregulated, it promotes regeneration of posterior structures. However, in Capitella it’s likely not that simple.”
By comparing expression of Wnt genes in fragments of cut worms, Kunselman said it looks like the regions where Wnt genes were turned on seem to differ between anterior and posterior fragments.
“This is significant,” Kunselman said, “because it could mean that Wnt genes have different roles in Capitella regeneration than they do in other organisms, which may mean that there are other ways regeneration is regulated.” This implies that there are multiple mechanisms of regeneration — good news for us vertebrates who have limited regenerative capacities as adults.
Kunselman intends to continue studying the role of Wnt genes in regeneration by disrupting their signaling to see how Capitella’s ability to regenerate changes. Researchers can use certain drugs to both disrupt and amplify Wnt signaling, sort of like disrupting or amplifying a Wi-Fi signal with a router in your home. If Wnt is crucial for regeneration, then jamming Wnt signals will prevent regeneration from occurring. Conversely, increased Wnt signals should induce regeneration.
“It is exciting to begin unraveling the molecular basis of regenerative abilities,” said Dr. Seaver. “It points to the importance of studying a wide range of animals with differing regenerative properties, including segmented worms.”
The research done by Boyd and Kunselman represents important early steps for understanding the basic biological principles of regeneration and restoring regenerative potential when and where it has been lost. The hope is to someday apply these principles to other species — maybe even humans — to help us regenerate tissues that don’t regenerate on their own. Someday in the future, if you have an unfortunate accident and cut your foot, you may be grateful for this little-known segmented marine worm that may help you grow back your little toe.